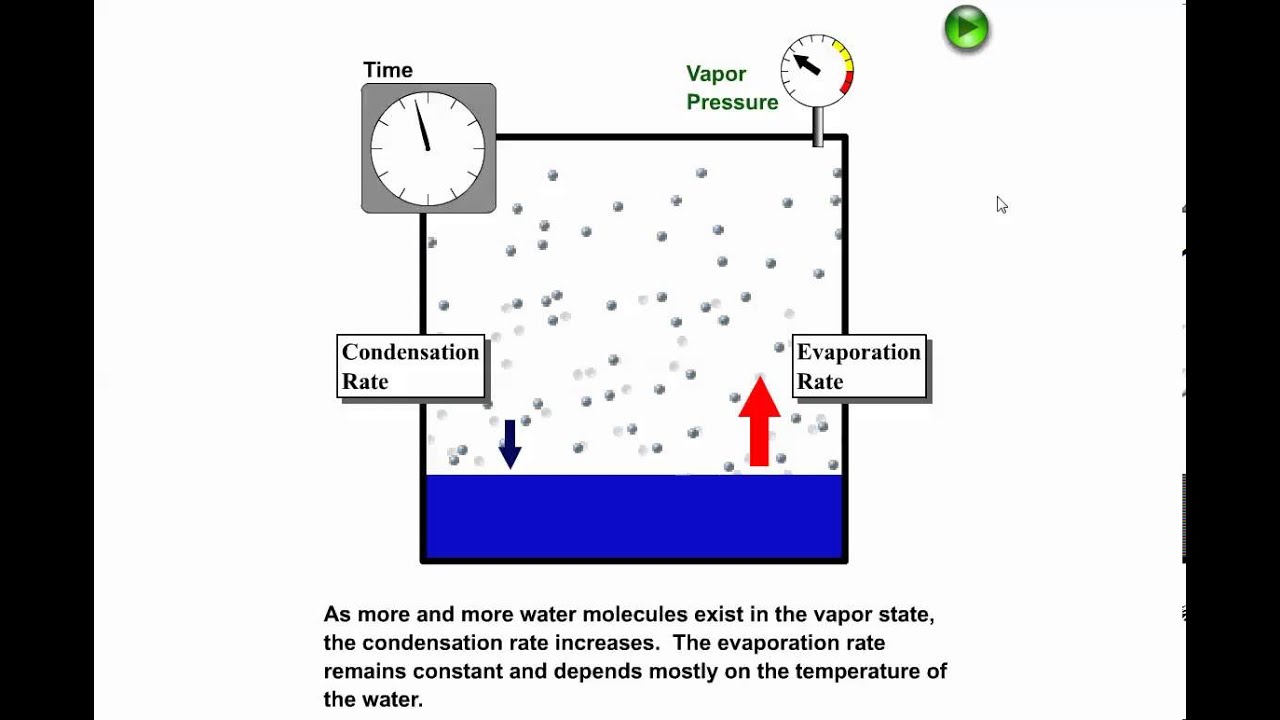
Because of the hydrogen bonding between water molecules water is a liquid at room temperature.
Why water is a liquid at room temperature while methane is a gas.
Water has nearly the same mass as methane and ammonia molecules but the greater molecular forces between water molecules causes the water to be liquid at room temperature while ammonia and.
Therefore at 78 degrees f carbon dioxide is above its boiling point while water is.
If possible explain with reference to their molecular structure shape and find homework help for.
While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas the gas at ambient temperature is lighter than air.
Get an answer for explain.
Room temperature is defined as anywhere from zero to 100 degrees celsius.
Molecules like oxygen gas and nitrogen gas are gases at room temperature.
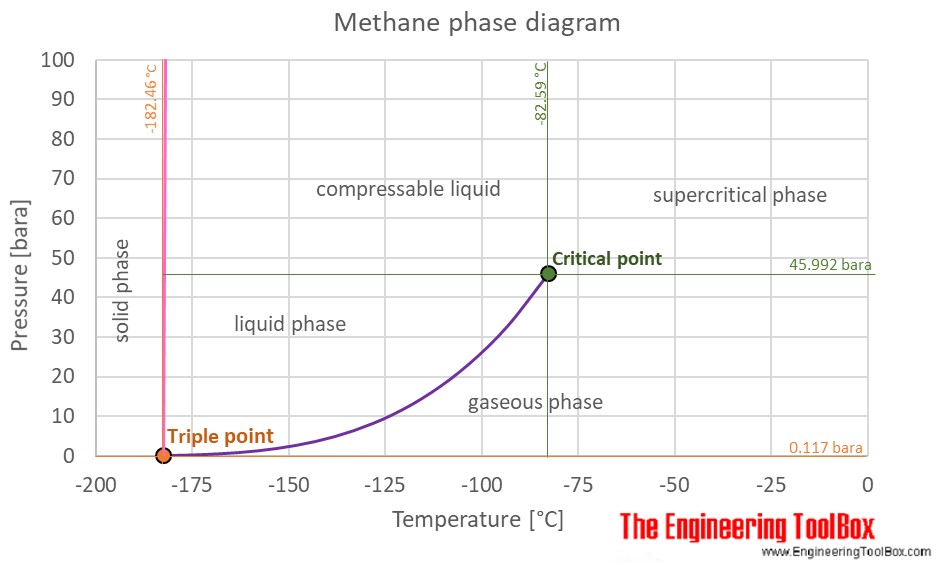
At room temperature methane ch4 is a gas but water is a liquid.
Methane is used in industrial chemical processes and may be transported as a refrigerated liquid liquefied natural gas or lng.
Water changes easily between its three forms.
Water becomes a gas when the hydrogen bonds that form molecules move quickly.
An overall sticky effect is created.
Carbon dioxide is a gas at room temperature while water is found primarily in liquid form at room temperature.