Saturated fats can stack themselves in a closely packed arrangement due to its carbon hydrogen composition and so they can freeze easily and are typically solid at room temperature.
Why are saturated fats solid at room temperature multiple correct answers.
It has to do with the fact that there are more hydrogen atoms in the saturated fatty acid molecules than in.
So they stay solid at room temperature.
One of the properties of saturated fats is that they are solid at room temperature.
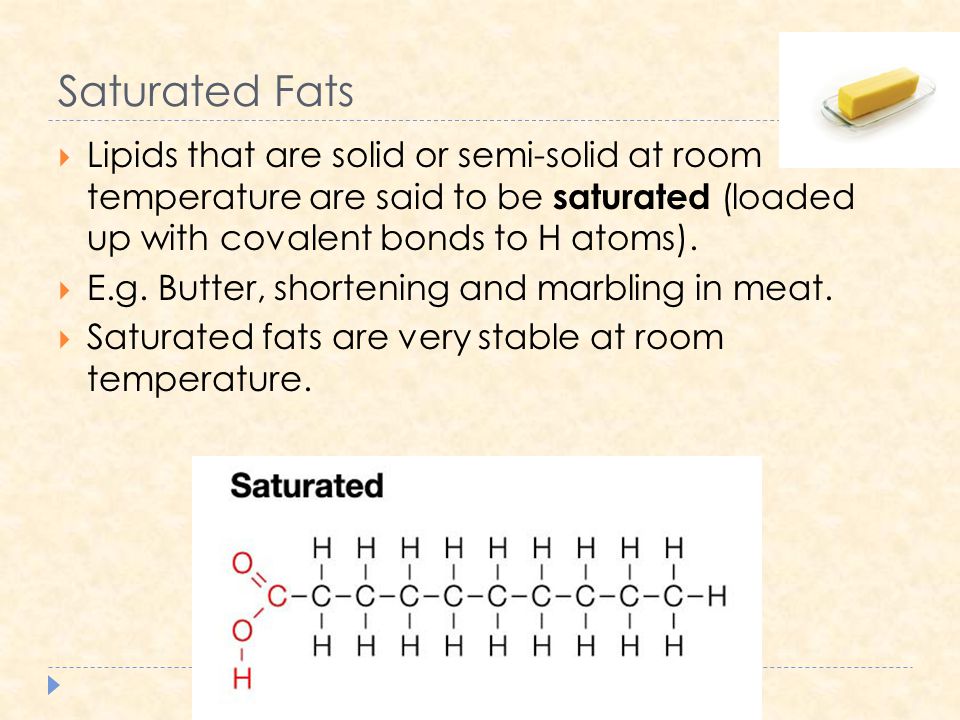
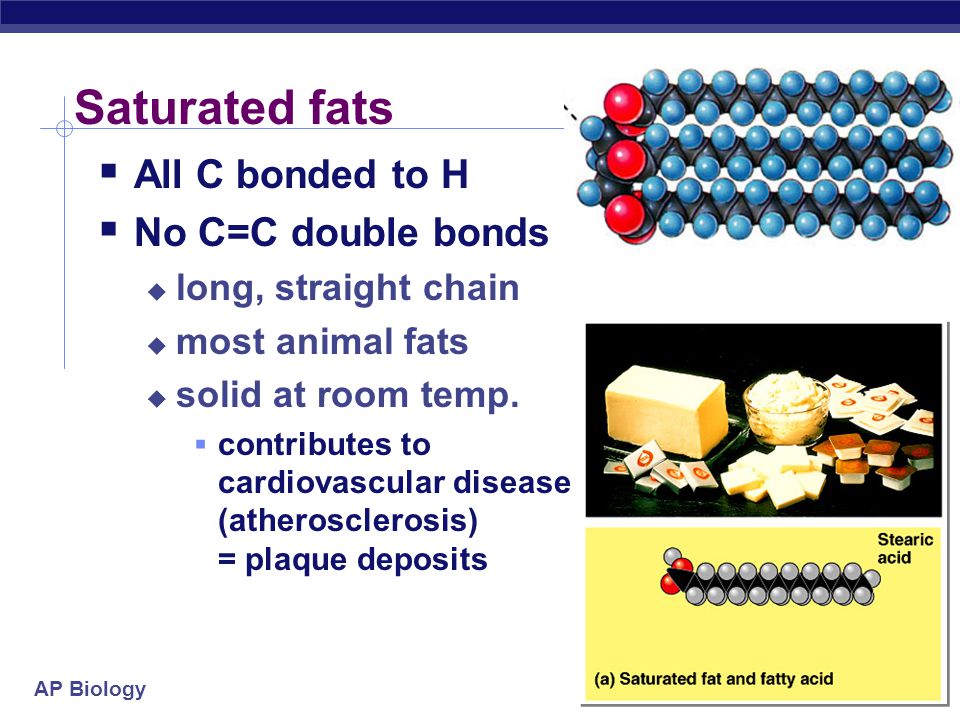
Fats that are tightly packed with no double bonds between the fatty acids are called saturated fats.
Because those carbon chains are so full with hydrogen atoms the chains are stiffer less flexible.
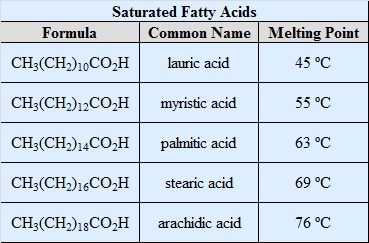
Fats triglycerides that contain palmitic acid and stearic acid are therefore known as saturated fats.
Imagine a building made of solid bricks.
This building of bricks is similar to the tightly packed bonds that make saturated fat.
Liquid at room temperature solid at room temperature structures containing only saturated fatty acids structures with equal proportions of unsaturated and saturated fatty acids.
C it makes the oil more solid.
Long chains of hydrocarbons form rods that pack tightly together forming a high density of intermolecular contacts.
A it makes the oil more saturated.
Two of the carbons are connected by a double bond and two of the hydrogens are missing.
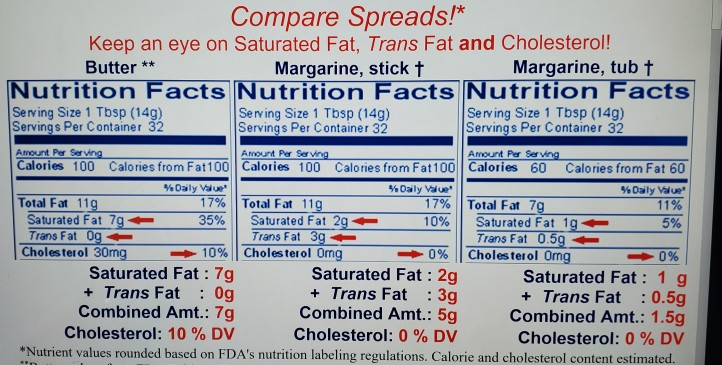
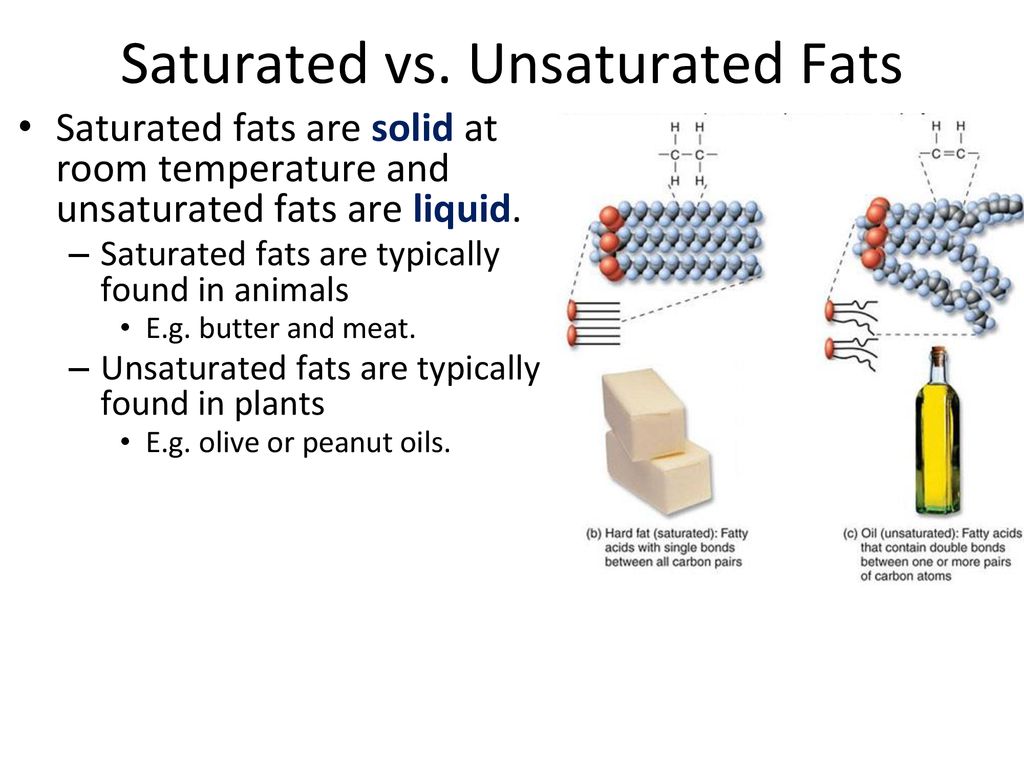
Saturated fats are most often found in animal products such as beef pork and chicken.
There are three main categories of fat.
Saturated monounsaturated and polyunsaturated.
A are generally solid at room temperature.
D it results in the formation of trans fatty acids.
This is the same principle that explains why long chain saturated hydrocarbons are solids at room temperature.
There are some exceptions but most are solid at room temperature.
Saturated fats are chains of carbon atoms with as many hydrogen atoms on that chain as possible.
All of the following are correct about unsaturated fats except.
Sources of saturated fat.
This formation is stable and due to this reason the melting temperature of these types of fats are increased.
You can also see that oleic acid is not saturated.
The fat that is solid at room temperature is saturated fat.
This fatty acid is unsaturated.
The main difference between fats and oils is that fats are.
It is beneficial to eat fats that are liquid at.
The bonds are often solid at room temperature like butter or the fat inside or around meat.
B are found only in animal products.
B it makes the oil healthier to consume.
This is why saturated fats are solid at room temperature think.